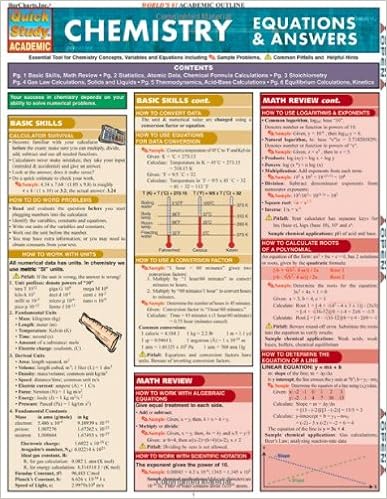
By Inc. BarCharts
This 6-page examine consultant includes uncomplicated chemistry research and ideas designed particularly to assist technological know-how students.
Read Online or Download BarCharts QuickStudy Chemistry Equations & Answers PDF
Best general & reference books
Theory of the stability of lyophobic colloids
This chemistry vintage bargains an exceptional, hugely proper account of the steadiness of lyophobic colloids and suspensions and develops a quantitative conception at the topic. significant subject matters encompass the speculation of a unmarried double layer (with concerns of the distribution of the electrical cost)
Chemistry of Heterocyclic Compounds: The Pyrimidines: Supplement II, Volume 16
Content material: bankruptcy I advent to the Pyrimidines (H 1, E 1) (pages 1–20): bankruptcy II The critical man made approach (H 31, E 20) (pages 21–62): bankruptcy III different tools of basic Synthesis (H eighty two, E fifty three) (pages 63–108): bankruptcy IV Pyrimidine and its C? Alkyl and C? Aryl Derivatives (H 116, e86) (pages 109–134): bankruptcy V Nitro Nitroso and Arylazopyrimidines (H 138, b ninety four) (pages 135–156): bankruptcy VI Halogenopyrimidines (H 162, E a hundred and ten) (pages 157–224): bankruptcy VII Hydroxy?
Turn into conversant in the fantastic global of atoms and molecules during this consultant written for readers who've little-to-no publicity to chemistry. The ebook offers an user-friendly creation to chemistry yet is usually used as an exceptional evaluation of the topic and discusses themes together with chemical reactions; the periodic desk of the weather; nuclear approaches; acids, bases, and salts; chemical bonding; environmental chemistry; and natural and biochemistry.
Analytical Pyrolysis. Techniques and Applications
1984 (this quantity is the results of lectures offered on the 5th foreign Symposium on Analytical Pyrolysis, held at Vail, Colorado), hardcover variation, Butterworths, London, U. okay. Hardcover name, 486 pages
- Underground Cable Thermal Backfill. Proceedings of the Symposium on Underground Cable Thermal Backfill, Held in Toronto, Canada, September 17 and 18, 1981
- Chemistry of Heterocyclic Compounds: Chemistry of 1,2,3-Triazines and 1,2,4-Triazines, Tetrazines, and Pentazin, Volume 33
- Organolithium Methods (Best Synthetic Methods Series)
- Colorants for Non-Textile Applications
- Ferrocenes Ligands Materials and Biomolecules
Extra resources for BarCharts QuickStudy Chemistry Equations & Answers
Example text
EtO HO ~N) ~NJ NH 2 (HO=EtO) MgI y I EtMgI H NH 2 -----+ Mel ~N) ~;S) Me Me HO~ EtO~ (hypotheticaO.... ~ Me Me eseroline HO~ ~N~~) Me 34 INDOLE ALKALOIDS Me ~ N Me 0 Me NH 2 EtOCCb Me Et0Ct:t' ~ N~O NH Me P20S Na/EtOH > ? H~el dl-eserethole Me N N Me N ~ N Me 0.. J eserethole methiodide Me Ie ~ --+ Me ~N~O Et00--YEt Me Me Htl1 ~ 2. Hofmann 3. H I. Mel N Me 2 HOG --+ Me N/ ~O Me NMe2 K3 Fe (CN)6 NMe2 OH eserethole methine N Me Me 37 SIMPLE DERIVATIVES OF TRYPTOPHAN NMe, The Harmala Bases. Harmine (the main base), harmahne and harmalol, along with the quinazoUne, vasicine, make u p about 2 - 3 % of the seeds of the rutaceous plant, Peganum harmala.
Pyrolysis of isoevodiamine hydrochloride evolved water, methyl chloride and by oxidation or disproportionation yielded rutaecarpine. Oxidation of evodiamine with potassium permanganate in acetone gave hydroxyevodiamine (a misnomer since it is a fcw-amide). Boiling these derivatives in alcoholic potassium hydroxide furnished j3-carboUne, tryptamine and anthranilic acid derivatives. 3. The synthetic approaches are for the large part reversals of the degradative steps but in an interesting variant o-aminobenzaldehyde was first coupled to 3,4-dihydro-j8-carbohne then oxidized.
5. The double bond must in some way facilitate the racemization of lysergic acid ^/-lysergic acid b u t n o t dihydrolysergic acid is racemized by heating it in baryta at ISO"" under nitrogen). 6). 6. If lysergic acid is pyrolysed fission typical of a jS-amino acid is observed resulting in a n unsaturated lactam. All doubts as t o the correctness of the structure of lysergic acid were removed by a synthesis first of all of rú[c-6,8-dimethylergoline and then rac-dihydrolysergic acid but the comparisons were made difficult because of the production of isomers (three asymmetric centres are involved).



